Investigating the effect of lean amine temperature and circulation rate on CO2 slip in MDEA-based Gas Sweetening plants
Omid Sabbagh, Maissam Vahidi Ferdowsi, and Mohammad A. Fanaei
Department of Chemical Engineering, Ferdowsi University of Mashhad Mashhad, Iran
Abstract
MDEA selectively removes H2S while allowing a large fraction of CO2 to slip through unabsorbed. MDEA has been used for selective H2S removal in various applications, including natural gas processing, Claus tail gas treating, and synthesis gas treating for integrated gasification combined cycle (IGCC) process. Nowadays, very large studies are done on relationship of sensitive parameters and CO2 slip. In this study, the effect of lean amine temperature and circulation rate simultaneously on CO2 slip is evaluated by using the simulation and industrial validation. The results demonstrate that the reducing of inlet amine’s temperature as much as possible and increasing amine circulation flow rate can increase the selectivity of MDEA and CO2 slip.
Keywords: CO2 slip, MDEA selectivity, lean amine temperature, amine circulation rate
Introduction
Many years ago, using aqueous amines to achieve a 4 ppm H2S specification in the treated gas was invariably accompanied by the nearly complete, simultaneous removal of CO2. Nowadays, the gas processing industry routinely requires that various amounts of CO2 are left in the gas, and demands that the treated gas meet ever more stringent quality specifications, especially in low pressure applications like tail gas treating. Another example is the treating of a gas stream with relatively low total acid gas content and a very high CO2 to H2S ratio where a higher than normally allowable CO2 content output stream can be blended with other gas streams. The amines that have been proved to be of principal commercial interest are MEA, DEA and MDEA. Generic methyl-di-ethanolamine is commonly used as a highly selective solvent to treat sour gases down to parts-per-million levels of H2S while slipping a large proportion of the CO2 in the feed gas from the system. Since, the CO2 reaction rate with MDEA is slow, the addition of small amounts of fast reacting amines is necessary to apply this process in natural gas treatment. Many studies were performed on the kinetics of the reaction of CO2 with aqueous MDEA [1, 2]. A comprehensive overview on that subject is provided by Vaidya and Kenig [3]. There is an agreement on the reaction mechanism which implies that tertiary amines do not react directly with CO2. In an aqueous solution, MDEA catalyzes the CO2 hydration reaction according to the mechanism proposed by Donaldson and Nguyen [4]. However, there are still many discrepancies in the literature concerning the interpretation of the kinetic data. This has brought about the use of new amines, the development of blended amine technology, use of special additives and process variables setting to enhance H2S removal.
In recent years, more research has been done in the area of CO2 slip increasing, is based on Ralph H. Weiland’s works. Weiland et al. (2001), studied on the relationship between tray/ packing hydraulics and CO2 rejection [5]. In another work (2003), they focused on development of fundamental understanding of how modern amine solvent technologies work and how MDEA achieve good CO2 slip [6]. In 2008, R. H. Weiland also modified the simple standalone AGE process with using a separate absorber to increasing CO2 slip [7]. Weiland et al. (2009) evaluated the limitations of using generic MDEA solutions for CO2 removal applications [8]. In 2011, they studied the effect of acid gas loading and MDEA solution flow rate on the CO2 slip according to operability limitations [9]. B. Spooner et al. (2012) investigated the effect of amine feed point into absorber, amine circulation rate, amine strength and lean amine temperature on MDEA selectivity specification separately [10].
As mentioned in literatures, most of studies are focused on effect of sensitive parameters individually. But this article evaluates the effect of lean amine temperature and circulation rate simultaneously on CO2 slip. In this investigation a commercial process simulator (Aspen HYSYS V. 8.3) is used for absorber column modeling and also a typical gas refinery is selected for validation of thermodynamic and kinetic models.
Foundation of MDEA Selectivity
CO2 does not react directly with the MDEA molecule; instead it dissolves and reacts in the water portion of the solution.
CO2 + H2O →H2CO3 (carbonic acid)
H2CO3 →H+ + HCO3- (bicarbonate)
H+ + R1R2R3N →R1R2R3NH+
CO2 + H2O + R1R2 R3N →R1R2R3NH+ + H
The reaction between CO2 and water (carbonic acid formation) is a “slow” step; it takes time to occur. Once the carbonic acid is formed, however, the MDEA reacts with it quite quickly and the bond will not be broken again until the amine is regenerated. The removal of CO2 with a tertiary amine like MDEA is therefore kinetically limited by the reaction rate in the first step.
H2S on the other hand, reacts directly with the MDEA molecule;
H2S + R1R2R3N →R1R2R3NH+ + H
The reaction between H2S and the amine is a very fast or instantaneous reaction. This means that H2S removal is almost always equilibrium limited. Each contact stage or tray in an absorber reaches the H2S equilibrium between gas and liquid. The difference in chemistry between the H2S and CO2 removal is the key to understanding how H2S can be removed with a minimum amount of CO2.
Absorption column simulation
The Aspen HYSYS 8.3 simulator is utilized for simulation procedure. In this simulation, for the prediction of the thermodynamic properties, ACID GAS Package is selected. To use the non-equilibrium model for the trays or packed bed separation included in Aspen HYSYS®(Rate-Based) because on this way, we can consider finite heat and mass transfer; therefore, the efficiencies of the trays/packed bed vary along the columns; as a consequence, the energies are more realistic than using an ideal model.
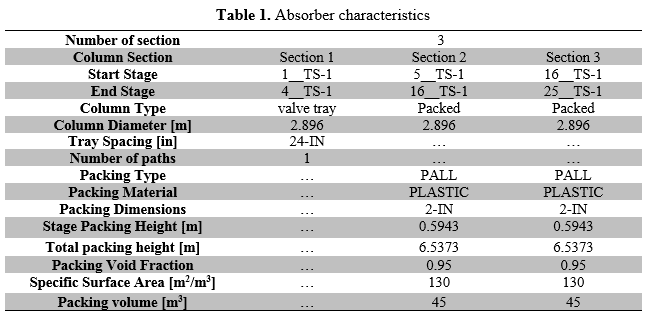
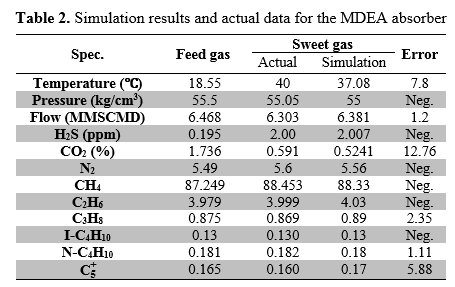
A typical Iranian gas plant (BIDBOLAND) is selected for this study. Gas sweetening facility has four identical amine trains for H2S and CO2 removal. Each train was composed of one packed bed absorber and one tray stripper columns, which operated in the unit. Absorption column operating conditions are shown in Table 1. The column feed and product composition and simulation results are summarized in Table 2.
Results and discussion
The analysis of acid gases composition in the unit product stream shows that increasing the flow rate of amine circulation to 950 gallons per minute decreases the level of hydrogen sulfide and carbon dioxide concentration in the sweet gas. This applies for each of the operating conditions and it is shown in figures 1 and 2. But as can be seen, increasing the amine circulation flow rate more than 950 gallons per minute, while the inlet amine temperature is 34°C, decreases the absorption level of carbon dioxide and increases its concentration in the sweet gas while the amount of hydrogen sulfide concentration in the sweet gas fall steadily.
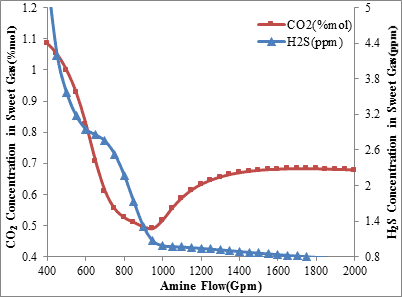
Figure1. H2S and CO2 concentration in sweet gas via amine circulation rate
(Inlet amine temperature is 34ºC)
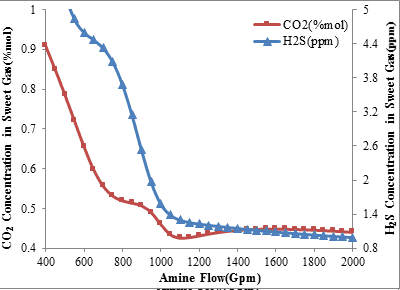
Figure2. H2S and CO2 concentration in sweet gas via amine circulation rate
(Inlet amine temperature is 45 ºC)
The reason for this may be the absorption mechanism of carbon dioxide by aqueous solution of MDEA. Since the absorption of CO2 by MDEA is controlled by the reaction kinetic, increasing the temperature leads to an increase in the reaction rate and more absorption of CO2. With this description, when the temperature of inlet amine stream is low and the amine circulation rate is high, the accumulation of reaction heat in the solution can not lead to significant increases in the temperature of the amine.
The comparison of amine temperature distribution curves with flow rate of 2,000 gallons per minute (figures 3 and 4) shows that when the input amine temperature is 34°C (Figure 3), the amine solution temperature never riches 40°C along the column but if the input amine temperature is increased to 45°C (Figure 4) the solution temperature will cross 50°C. According to what was stated, it seems that the minimum temperature required to initiating the reaction of MDEA solution with carbon dioxide in this case, is more than 40°C.
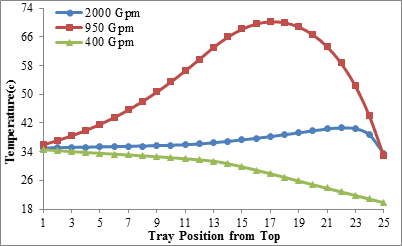
Figure3. Amine temperature distribution along the column for three levels of
amine circulation rate (Inlet amine temperature is 34 ºC)
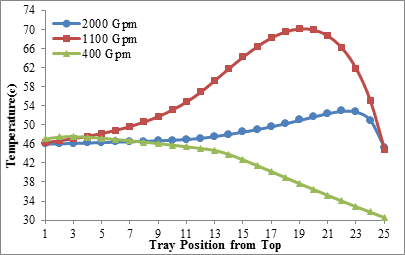
Figure4. Amine temperature distribution along the column for three levels of
amine circulation rate (Inlet amine temperature is 45 ºC)
The temperature changes of the rich amine and its relationship with the amine circulation rate is shown in figures 5 and 6. As expected, the maximum temperature appears at the point that most CO2 absorption occurs and the greatest heat of reaction is released. Based on this, when the CO2 removal is not important, the reducing of inlet amine’s temperature as much as possible and increasing amine circulation flow rate above the normal level, can increase the selectivity of MDEA and CO2 slip.
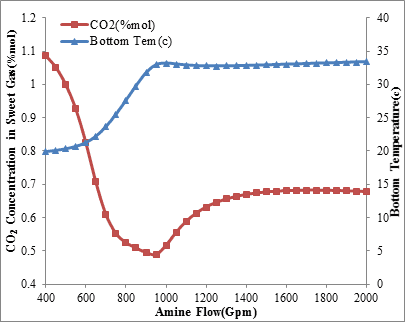
Figure5. CO2 concentration in sweet gas and rich amine temperature via
amine circulation rate (Inlet amine temperature is 34 ºC)
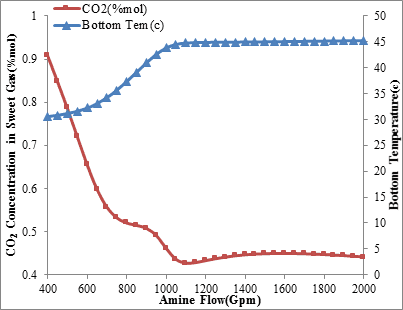
Figure6. CO2 concentration in sweet gas and rich amine temperature via
amine circulation rate (Inlet amine temperature is 45 ºC)
Conclusion
Attaining the maximum selectivity for H2S over CO2 is achieved by using the right solvent under the right process conditions in the right equipment. To this aim, this investigation focused on estimating the optimum process conditions in order to increasing CO2 slip. The effect of changing lean amine temperature and amine circulation flow rate on MDEA selectivity was simulated and analyzed in this work, by the process simulation software Aspen HYSYS (V 8.3) and industrial validation. The results showed that the CO2 slip was found to increase with increasing amine flow rate above the normal level and decreasing inlet amine temperature simultaneously.
Acknowledgments
This project was carried out within the Research stream on optimization studies of BIDBOLAND gas refinary. The authors wish to thanks the refinery research unit officials for their valuable contributions, financial support of this research project and providing all necessary operating information.
References
- Haimour, N.; Bidarian, A.; Sandall, O. Kinetics of the reaction between carbon dioxide and methyldiethanolamine. Chem. Eng. Sci. 1987, 42, 1393-1398.
- Benamor, A.; Aroua, M. K. An experimental investigation on the rate of CO2 absorption into aqueous methyldiethanolamine solutions. J. Chem. Eng. 2007, 24, 16-23.
- Vaidya, P. D.; Kenig, E.Y. CO2-alkanolamine reaction kinetics: A review of recent studies, Chem. Eng. Tech. 2007, 30, 1467-1474.
- Donaldson, T. L.; Nguyen, Y. N. Carbon dioxide reaction and transport into aqueous amine membranes. Ind. Eng. Chem. 1980, 19, 260-266.
- Weiland, R. H.; Dingman, J. C. How to Increase CO2 Laurence Reid Gas Conditioning Conference, Norman, OK, 2001.
- Weiland, R. H.; Sivasubramanian, M. S.; Dingman, J. C. Effective Amine Technology: Controlling Selectivity, Increasing Slip, and Reducing Sulfur. Laurence Reid Gas Conditioning Conference, Norman, OK, 2003.
- Ralph, H.; Weiland, R. H .Acid gas enrichment—maximizing selectivity. Laurence Reid Gas Conditioning Conference, Norman, Ok, 24–27 February, 2008.
- Seagraves, J.; Weiland, R. H. Treating High CO2 Gases with MDEA. Petroleum Technology Quarterly GAS, pp. 103-109, 2009.
- Weiland, R. H.; Hatcher, N. A. Stable Operating Limits in Amine Treating Units. Laurence Reid Gas Conditioning Conference, Norman, Ok, 2011.
- Spooner, B.; Derakhshan, F. Reduce CO2 in acid gas from amine-based TGTUs. Hydrocarbon processing. 2012.







No comment